News & Views, Volume 51 | Combustion Turbine Compressor Hygiene
COMPONENT LONGEVITY
By: John Molloy
Introduction

Figure 1. Compressor rotor inlet with fouling deposits
An industrial combustion turbine can ingest over 1000lbs of air per hour of operation. Entrained within the air is a spectrum of mineral, salt, moisture, and VOC, and other compounds that are present in the local atmosphere. Locally high concentrations of potentially corrosive species may also be present due to surrounding industries or even effluent from the power plant itself, such as cooling tower drift, evaporation cooler deposits, or water treatment effluent.
In addition to disrupting the flow path area of the compressor blades and vanes, with a consequential drop in compressor efficiency, these contaminants can also serve as sites for under-deposit corrosion cells that have implications for component life as well as risk for catastrophic failures. Compressor waterwashing with detergents has been utilized with some success by utilities as a method for mitigating the effects of deposit accumulation. Nevertheless, tenacious deposits can accumulate over time. The presence of moisture in the deposit can also result in activation of a corrosion cell that can corrode the typical stainless steels used for blade and vane construction. Higher strength PH stainless steel blades and vanes suffer a larger loss in fatigue endurance limit from pitting, and tend to suffer more airfoil liberations due to cracking initiated at pitting.

Figure 2. Forward compressor blade with leading edge pitting
On forward compressor blades and vanes, qualified inspectors have also observed combined effects of leading edge erosion and pitting. The erosion is typically the result of on-line water washing. The roughness of the eroded leading edge is now an ideal area for compressor deposits to become deeply embedded. Additionally, it serves as multiple stress concentrations for fatigue crack initiation.
The fall in compressor efficiency, the irreversible damage due to erosion and corrosion pitting, and the risk of catastrophic damage due to fracture initiation at the affected areas, all suggest that O&M staff need to have a strategy to mitigate compressor deposit accumulation, as well as erosion channeling. Additionally, the strategy should also consider the scenario where some deposit accumulation is unavoidable and how to reduce the activation of these deposits. Consideration should also be given to units that have a history of blade failures due to a design limitations that makes them susceptible to corrosion fatigue cracking. In such cases, the unit has a low tolerance to the presence of corrosion pitting for crack initiation.

Figure 3. Fractured forward compressor blade with leading edge pitting at the origin
Typical Sources of Contamination – Land
Local geology and soil can contain large amounts of calcium, iron, magnesium, aluminum potassium, sodium, phosphorus, sulfur, as well as other less common species, including chlorides where the dry land used to be a sea bed. These elements are usually present as a compound, such as a mineral oxide or a salt, but may also be bound with organic material in the soil. Even with reasonably high efficiency filtration that removes 99.7% of the particulates within a given particle size range, the remaining 0.3% contamination multiplied by 1000lbs /hr of influent air results in a high rate of deposit accumulation. Deposits accumulated on airfoil surfaces can then liberate ionic species due to the absorption of moisture, thus creating a corrosion cell. Chlorides due to ubiquitous salts will rapidly corrode (pit) any and all of the stainless steels used in gas turbine compressors, with only partial mitigation by coatings. The only class of compressor materials reasonably immune to chloride or under deposit corrosion is the titanium alloy blades and vanes used in flight turbines and some aeroderivative units. Sulfur containing compounds are also commonly found on the airfoils of compressor blades and vanes, particularly in regions with heavy industry and refining. Sulfur containing compounds can also be extracted and diverted to the hot gas path cooling channels of downstream components, and greatly accelerate hot corrosion in the absence of moisture.
Atmosphere
The local quality of air entering the compressor is somewhat variable, and also dependent on geographic location. Coastal regions have an obvious problem with humid, chloride laden atmosphere. This particular environment is especially vulnerable to chloride pitting. Special inlet ducting and tailored filtration may provide some level of protection.

Figure 4. Fractured forward compressor blade with leading edge erosion at the origin

Figure 5. Forward compressor blade with leading edge erosion at platform radius
Local environments due to the proximity of inlets relative to cooling towers and the use of sodium hypochlorite for microbial control of cooling tower water can result in a chloride-laden influent to the unit. In this case the solution may be as simple as using a non-chloride based biocide for microbial control. In some cases the corrodents can come from non-typical activities at the plant, such as excursions of acid vapors from the water treatment facilities (sulfuric acid or hydrochloric acid).
Proximity to local industry, such as steel mills, coal or lignite fired boilers, oil production or refineries can result in a large influent concentration of sulfur bearing compounds. Sulfur bearing compounds, when incorporated into a deposit layer, can accelerate under-deposit corrosion and pitting, as well as aforementioned hot corrosion of downstream turbine components.
Water
Water source used for compressor washing (online and offline), as well as water used for power augmentation (misting, evaporative cooling, etc) should ideally be demineralized quality or better. The use of city water or another source of hard water is generally discouraged. Online water washing with a hard water source can result in increased deposit accumulation at and beyond the phase transition area (stage 2-5 typically) where the water boils to vapor and the dissolved minerals will deposit onto the blade /vane surfaces. Power augmentation by closed loop chiller systems also affords the opportunity for contamination due to leaks in the chilled fluid. These fluids can have variable water quality as well as chemicals added to the chiller loop. Non volatile constituents of the chilled water may leave deposits in a similar fashion as hard water. Evaporative cooling with hard water will result in mineral shedding that can accumulate downstream. As mentioned, on-line water washing will often cause leading edge erosion channeling, and more severely if the droplet size is not controlled, or leaking occurs during operation.
Historical Contaminants Observed in Compressor Deposits
Over the course of many years, we have been privileged to sample the deposits laden on blades and vanes from gas turbine units in many parts of the world. A pattern of contaminants (the usual suspects) has been observed, with some exceptions. The deposits observed are biased largely by one of the dominating contributors: Coastal conditions, land conditions, or local plant and industrial environment (neighboring industry or locally produced effluent).

Figure 6. Forward compressor blade with leading edge erosion at airfoil mid span
Energy Dispersive Spectroscopy (EDS) provides qualitative elemental analysis of materials under scanning electron microscopy (SEM) examination based on the characteristic energies of x-rays produced by the electron beam striking a deposit sample. The relative concentrations of the identified elements are determined using semiquantitative, standardless quantification (SQ) software. This information can be used to tailor the filtration system and media, as well as the detergents used in compressor washing. It may also provide leverage for high level dialog with the local regulators and emitting sources.
Conclusions
The corrosion pitting on the compressor blades and vanes is almost always the result of under-deposit corrosion aggravated by the presence of corrosive species in the deposit. The corrosive species are often sulfur and chlorine-containing compounds, but pitting can also occur from simply the presence of oxygen under the deposit (oxygen pitting). The pitting is proof of corrosive deposits, and trace amounts of corrosive species identified by EDS at the bottom interface of the pits identifies the active corrodents so that treatment can be fine-tuned for that species.
The source of the corrodents can be local to the plant (cooling tower drift), in the soil, from the atmosphere (coastal chlorides) or from local industry (burning lignite or low grade coal, and / or oil and gas production). In some cases the corrodents can come from non-typical activities at the plant, such as the use of sodium hypochlorite (bleach) for biological control, or excursions of acid vapors from the water treatment facilities (sulfuric acid or hydrochloric acid). Plant operations can identify, address and mitigate the local sources.
Starts-based units, or peakers, also have life-limiting pitting that is difficult to understand without considering the effect of off-line corrosion. For units operating in cycling duty, a substantial amount of time is spent in idle mode. During idle periods, the unit is normally stationary or on turning gear for some daily period. After the rotor is ambiently cooled during the nightly lows, the rotor will retain much of the lower temperature relative to the increasing ambient temperature and humidity during the day. As a result the rotor will sweat like cold drink on a warm day, and the deposits on the blades and vanes will be similarly affected. This is a common mechanism for deposit activation and corrosion that seems to be aggravated by the new paradigm of unit cycling. These are the periods where corrosive deposits combined with moisture will create conditions ideal for pitting, particularly if the airfoil deposits contain a substantial concentration of sulfur and chlorine-containing compounds.
Mitigation
Units may be waterwashed online each day when operated and the ambient temperature is greater than about 50°F. Offline water washing may be performed prior to performance testing or to restore lost capacity. In both cases the water washing is performed to provide performance benefits and also to prevent the accumulation of corrosive deposits. However, performing an online waterwash prior to operation does not remove the deposits accumulated during the subsequent operation cycle, nor does it remove much deposit beyond the second stage due to phase transition from liquid to vapor. Moreover, if the subsequent operation cycle is followed by a long idle period in humid weather, the deposits can absorb the ambient moisture and activate the corrodents. Off-line washes must be performed with demineralized water and a cleaning solution tailored to the deposits. Proper rinses with conductivity measurements taken at the drain ports are advised. Offline water washing is much more effective at removing compressor deposit accumulation, but proper drying is necessary to prevent pitting under remaining, tenacious deposits.
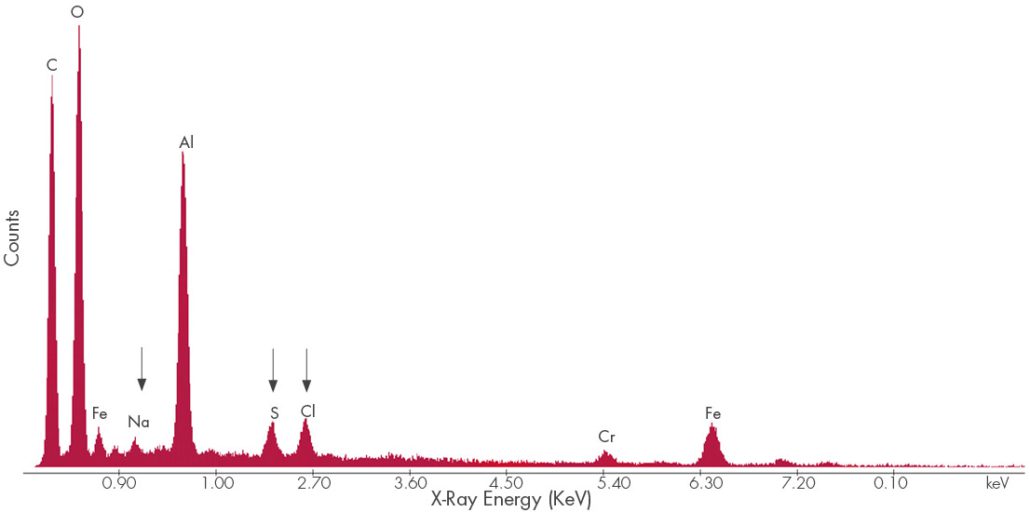
Figure 7. EDS Spectrum of compressor fouling deposits. Chlorides, sulfur deposits, and sodium deposits can be corrosive to stainless steels.
Another aspect of the operation that can affect the compressor deposits and moisture is proper sealing of the inlet filter house. All seams should be sealed with a weatherproof material and the fitment surfaces should be in good condition. Water leaks from the roof or any other area that causes standing water should be addressed. Additionally, corroded surfaces should be prepped and painted to stop the corrosion. Any bolts, nuts, and screws should be inspected for significant corrosion and potential loss of material that can become foreign object damage.
From a mitigation standpoint, two aspects must be addressed to reduce the susceptibility to pitting. First, the accumulation of deposits must be reduced. This may be achieved by higher efficiency filtration media (such as HEPA and hydrophobic Gore® Filters) and more aggressive offline water washing with a cleaning solution tailored to the deposits. Second, the presence of unwanted water must be reduced. Waterwashing should be followed by operation to ensure all moisture is evaporated. Water repelling filtration (such as Gore® filtration media) may reduce some of the water ingestion during wet weather, and may also prevent some of the influent from cooling tower drift (Gore® filtration medial is quite expensive, so a cost-benefit analysis may be require do to justify the additional expense). Long idle or layup periods should be combined with closure of the bellmouth and with a dry air source (heater or dehumidifier) to ensure that corrosive deposits are not activated. Mist eliminators and auto-close stack dampers are also beneficial, and should have some effect on reducing the ingress of moisture into the combustion turbine unit.
A detailed unit inspection may be advised, during which time deposit samples can be collected from the pre-filters, conical filters, and forward compressor blades / vanes / IGVs for corrosive species survey, as well as establishing the efficacy of the current filtration system. At this time, the severity of any pitting can also be documented. Additionally, mold replication can be performed on the LE of the forward compressor blades to gauge the severity of erosion channeling, which is often governed by OEM technical letters or service bulletins. A survey of the inlet filter house is also advised, where potential points of uncontrolled ingress can be identified. Additionally, the structure can be mapped for corrosion, cracked or damaged structures, and potential fastener liberation.



